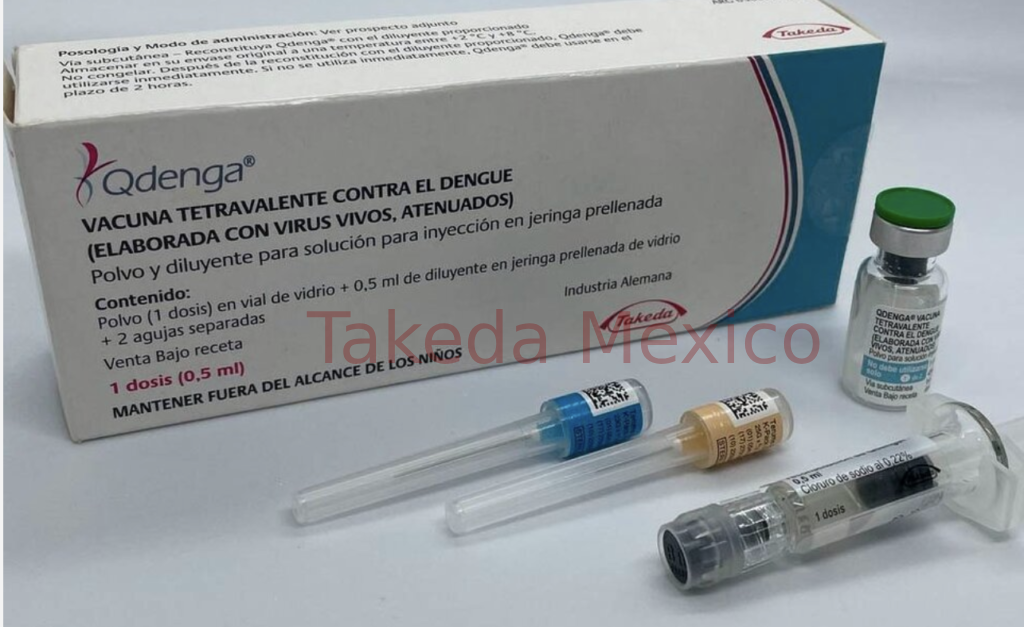
Dengue Tetravalent Vaccine
| Product Overview | |
| Generic Name | Dengue Tetravalent Vaccine |
| Brand Name(s) | QDENGA / TAK-003 |
| Form | Single dose vial for Sub-C |
| Strength | 0.5mL |
| Therapeutic Class | Viral vaccine, live attenuated (prophylactic) |
| ATC Code | J07BX04 |
| Manufacturing & Regulatory | |
| Manufacturer | Takeda |
| Country | Germany |
| GMP Compliance | WHO-GMP |
| DMF/CEP | Not publicly disclosed |
| COFEPRIS | Registered in Mexico, Batch-specific specific clave unknown |
| Free Sale Certificate | Yes |
| Logistics & Export | |
| MOQ | 5,000 units |
| Shelf Life | 18 to 24 months |
| Storage | 2–8 °C, protect from light |
| Incoterms | EXW/FOB/CIF negotiable |
| Lead Time | 60 to 90 days |
| Documentation | |
| Certificate of Analysis (COA) | Supplied per batch upon request |
| SDS | Upon Request, not publicly posted |
| CTD Summary | CTD available to regulators; summary available to qualified partners |
Description
Live, attenuated, tetravalent dengue vaccine designed to induce immunity against DENV-1, DENV-2, DENV-3, and DENV-4.