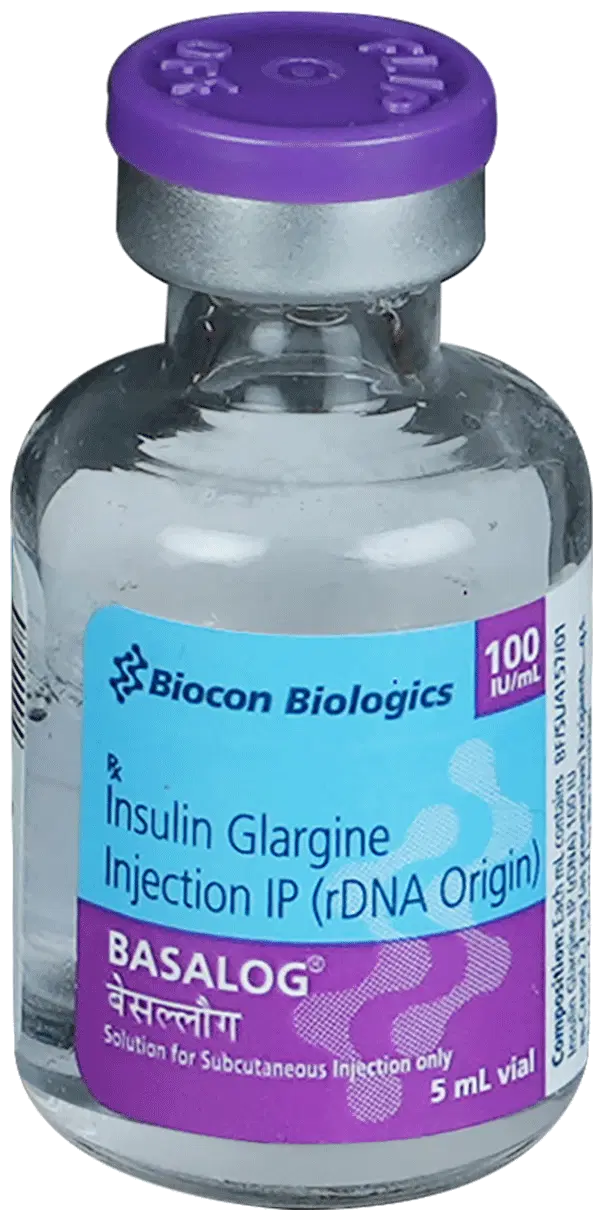
Insulin Glargine (Biosimilar)
| Product Overview | |
| Generic Name | Insulin Glargine (Biosimilar) |
| Brand Name(s) | Basalog, Basalog One, Basaglar, Lantus |
| Form | Vial |
| Strength | 100 IU\mL |
| Therapeutic Class | Antidiabetic agent; long‑acting insulin analogue |
| ATC Code | A10AE04 |
| Manufacturing & Regulatory | |
| Manufacturer | Biocon Limited |
| Country | India |
| GMP Compliance | WHO-GMP, EU-GMP |
| DMF/CEP | 27581 |
| COFEPRIS | Under Registration (2025) |
| Free Sale Certificate | Yes |
| Logistics & Export | |
| MOQ | 5,000 units |
| Shelf Life | 24 Months |
| Storage | 2–8 °C in fridge; do not freeze; |
| Incoterms | Ex-Works Mexico |
| Lead Time | 7 - 10 Business Days |
| Documentation | |
| Certificate of Analysis (COA) | Yes |
| SDS | Upon Request |
| CTD Summary | Upon Request |
Description
Indications & Usage: Once-daily basal insulin to improve glycemic control in adults and children (>2 yrs) with type 1 or type2 diabetes mellitus.