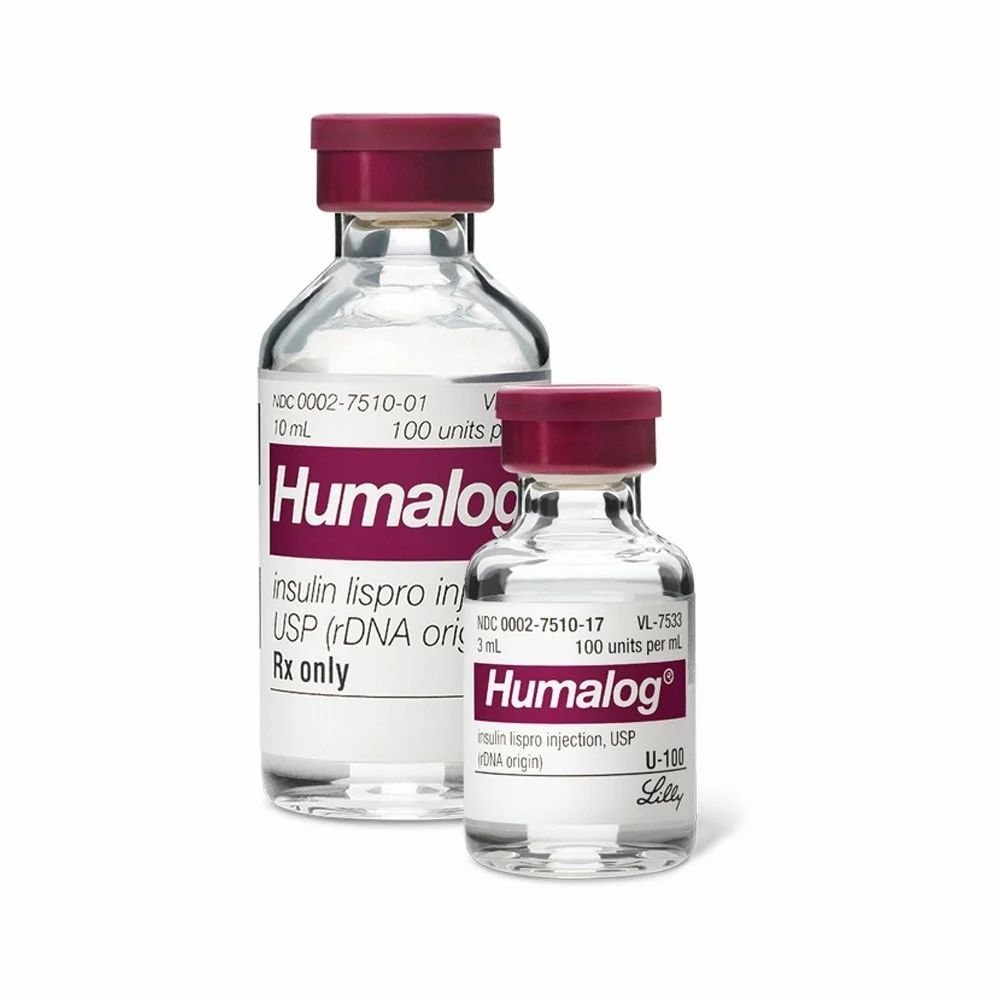
Insulin Lispro
| Product Overview | |
| Generic Name | Insulin Lispro |
| Brand Name(s) | Humalog |
| Form | Vial 10mL |
| Strength | 100iu/mL |
| Therapeutic Class | Antidiabetic; Rapid‑acting insulin analogue |
| ATC Code | A10AB04 |
| Manufacturing & Regulatory | |
| Manufacturer | Eli Lilly and Company India Pvt Ltd |
| Country | Manufactured in Lilly’s WHO-GMP‑certified Indian facilities. |
| GMP Compliance | WHO-GMP |
| DMF/CEP | Not publicly disclosed |
| COFEPRIS | Under Registration (2025) |
| Free Sale Certificate | Yes |
| Logistics & Export | |
| MOQ | 5,000 units |
| Shelf Life | 24 - 36 months |
| Storage | Store at 2–8 °C (36–46 °F); do not freeze |
| Incoterms | Ex-Works Mexico |
| Lead Time | 7 - 10 Business Days |
| Documentation | |
| Certificate of Analysis (COA) | Yes |
| SDS | Upon Request |
| CTD Summary | Upon Request |
Description
Indication/Usage: Rapid preprandial insulin to control post‑meal hyperglycemia in type 1 and type 2 diabetes; available in prandial and premixed formats.
- Recombinant human insulin analogue (biosynthetic)