Ustekinumab
| Product Overview | |
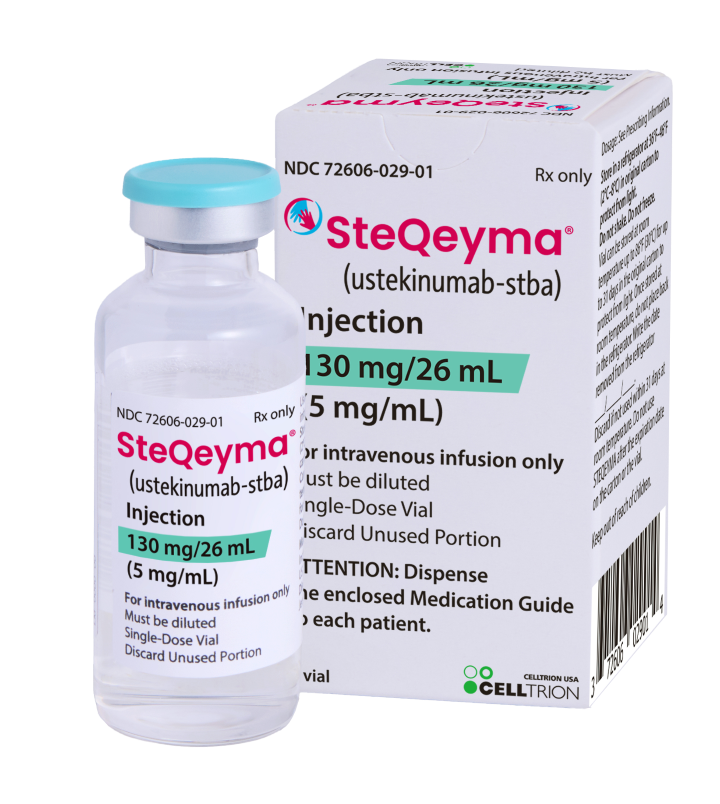
| Generic Name | Ustekinumab |
| Brand Name(s) | Stelara®, Ustekirel |
| Form | Intravenous concentrate for infusion (vial) |
| Strength | 130 mg/26 mL (5 mg/mL after dilution) |
| Therapeutic Class | Interleukin-12/23 pathway inhibitor (immunosuppressant) |
| ATC Code | L04AC05 |
| Manufacturing & Regulatory | |
| Manufacturer | Janssen-Cilag International NV., Real Life Sciences |
| Country | Belgium, Netherlands |
| GMP Compliance | WHO-GMP |
| DMF/CEP | U.S. approvals are via BLAs (125261 and 761044) |
| COFEPRIS | 010.000.5695.00 / 010.000.5695.01 |
| Free Sale Certificate | Available per batch upon request |
| Logistics & Export | |
| MOQ | 10 Units |
| Shelf Life | 24 Months |
| Storage | 2–8 °C, protect from light |
| Incoterms | EXW/CIF negotiable |
| Lead Time | 7 - 10 Business Days |
| Documentation | |
| Certificate of Analysis (COA) | Supplied per batch upon request |
| SDS | GHS-compliant SDS |
| CTD Summary | Public CTD-derived summaries via EMA EPAR/SmPC |
Description
Ustekinumab is a fully human IL-12/23 inhibitor indicated for plaque psoriasis (≥6 years), psoriatic arthritis (≥6 years), Crohn’s disease (adults), and ulcerative colitis (adults).