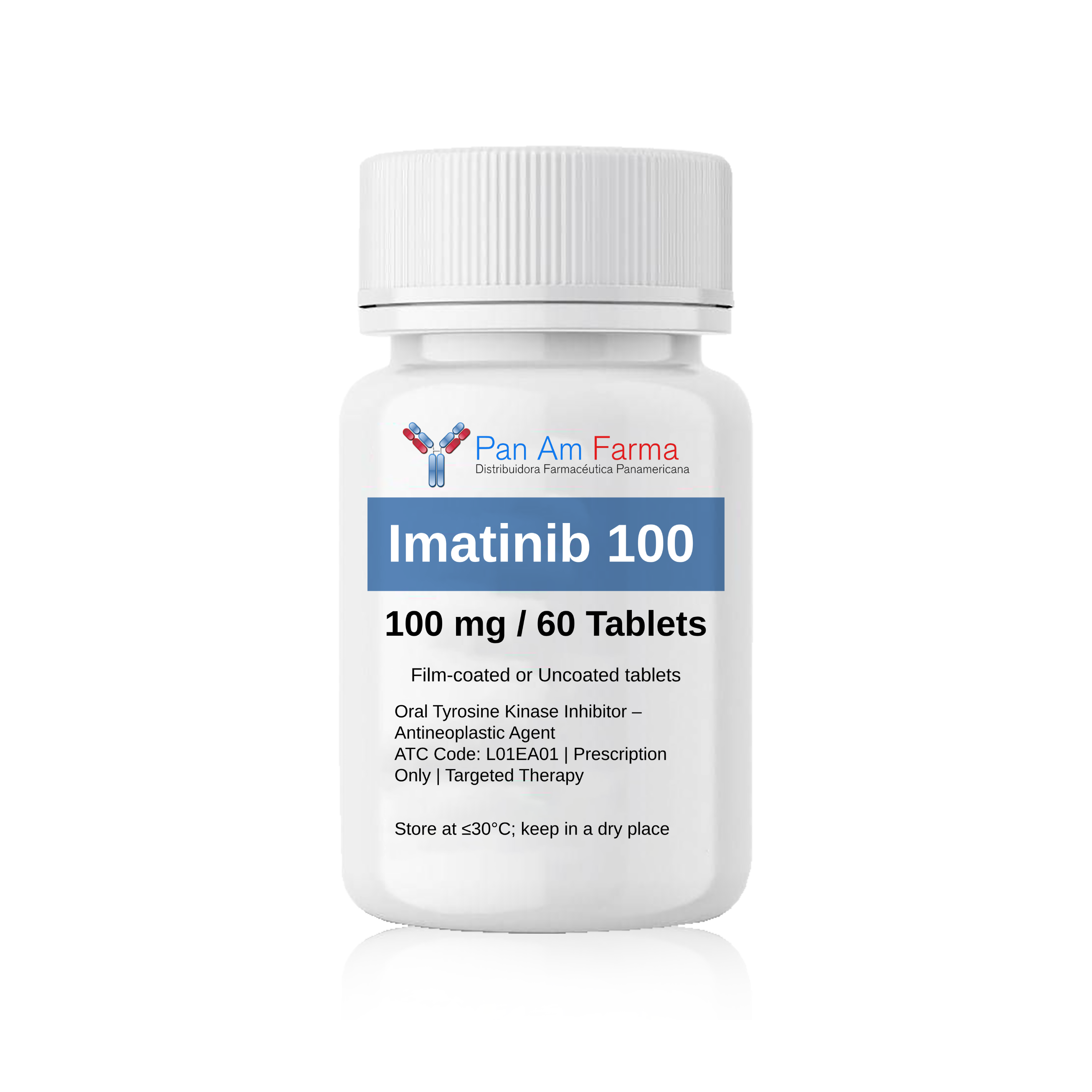
Imatinib 100 mg 60 Tablet
Si Disponible: Dispensado al Día Siguiente
Envío Gratis
Dentro de México

Requiere Receta Médica
Este Medicamento requiere prescripción médica de su médico, clínica, hospital o terapeuta.
Imatinib
Marca de Patente: GLEEVEC
Clase:
Es un inhibidor selectivo de tirosina quinasa (TKI)
Cómo Suministrado:
-Pastillas orales de mesilato de imatinib: 100 mg 60 Tab
Clave: 010.000.4225.00
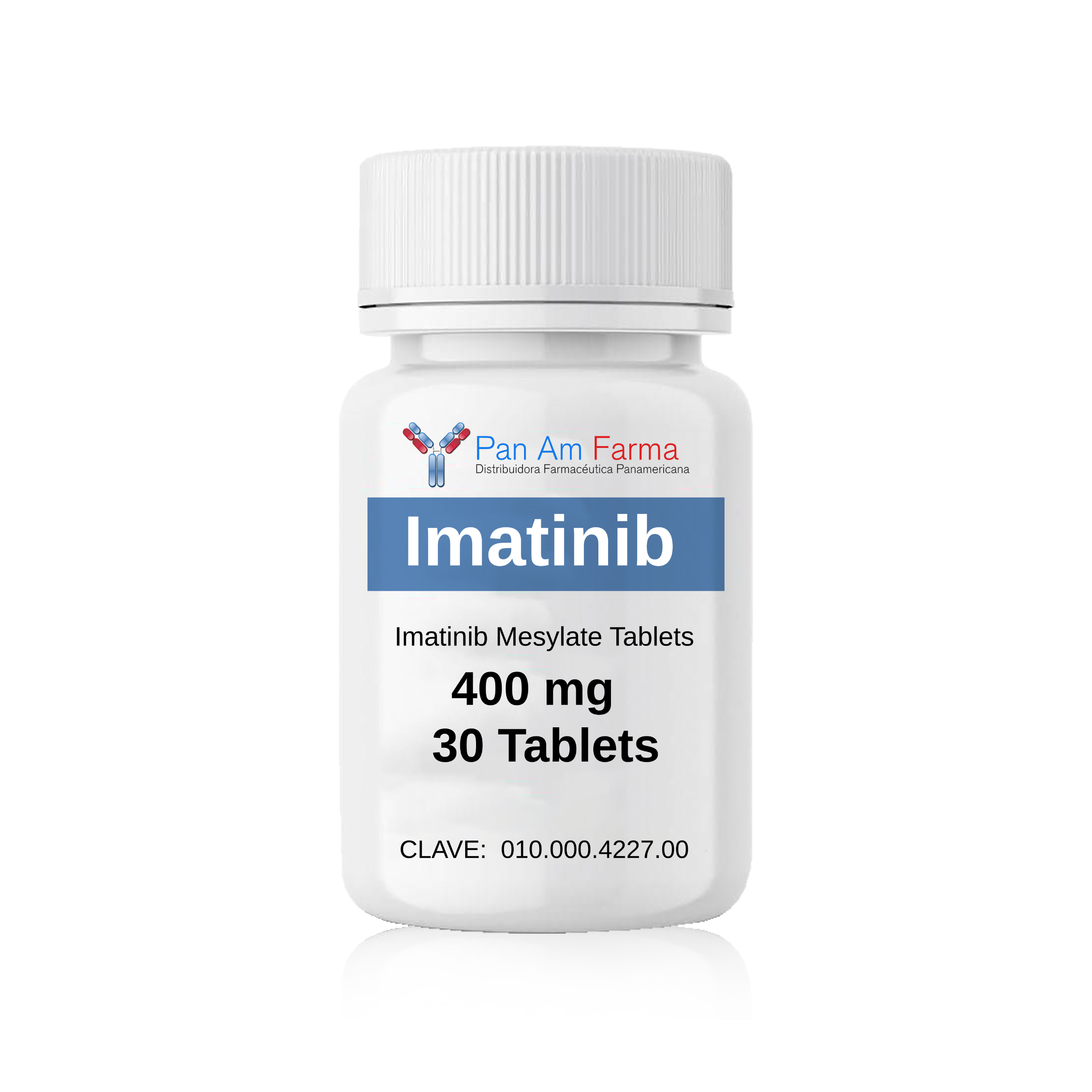
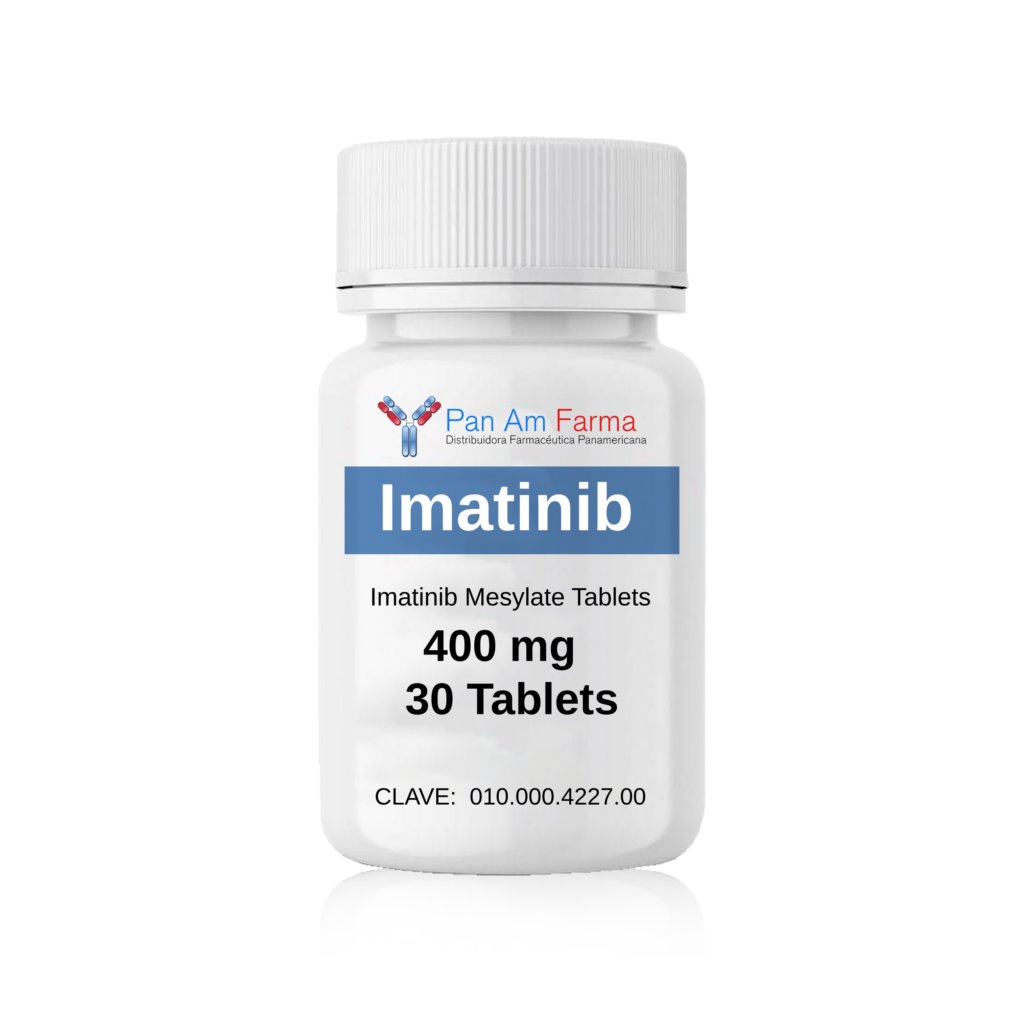
-Pastillas orales de mesilato de imatinib: 400 mg 30 Tab
Clave: 010.000.4227.00
DESCRIPCIÓN:
Indicado para el tratamiento de diversas neoplasias hematológicas y tumores sólidos, incluyendo leucemia mieloide crónica (LMC) y tumores del estroma gastrointestinal (GIST).
Description
IMATINIB 100 mg Tablets
Oral Tyrosine Kinase Inhibitor – Antineoplastic Agent
ATC Code: L01EA01 | Prescription Only | Targeted Therapy
Product Description:
Imatinib mesylate is a selective tyrosine kinase inhibitor (TKI) that specifically blocks the activity of BCR-ABL (the fusion protein associated with the Philadelphia chromosome), c-KIT, and PDGFR. It is indicated in various malignancies driven by abnormal tyrosine kinase signaling, most notably in Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML) and gastrointestinal stromal tumors (GIST). The 100 mg formulation is commonly used in dose-adjusted pediatric regimens and for patients requiring lower or divided daily doses.Available Presentation:
- Strength: 100 mg per tablet
- Formulation: Film-coated tablet (color and shape may vary by manufacturer)
- Packaging: Bottle or blister pack of 30, 60, or 90 tablets
- Route of Administration: Oral
- Storage Conditions: Store at ≤30°C; keep in a dry place
Uses:
- First-line and maintenance therapy in Ph+ CML (all phases: chronic, accelerated, blast crisis)
- Pediatric use in children with Ph+ CML
- Ph+ acute lymphoblastic leukemia (ALL) in combination with chemotherapy
- Unresectable or metastatic GIST expressing c-KIT (CD117)
- Other off-label or approved indications based on mutation analysis, including:
- Hypereosinophilic syndrome (HES)
- Chronic eosinophilic leukemia (CEL)
- Systemic mastocytosis
- Dermatofibrosarcoma protuberans (DFSP)
Indications:
- Chronic Myeloid Leukemia (CML): Adults and children with Ph+ CML in all disease phases
- Acute Lymphoblastic Leukemia (Ph+ ALL): Newly diagnosed adults and children, in combination with chemotherapy
- Gastrointestinal Stromal Tumor (GIST): Patients with unresectable or metastatic KIT-positive tumors
- Systemic Mastocytosis and HES/CEL: In patients with mutations responsive to imatinib
- Dermatofibrosarcoma Protuberans (DFSP): Unresectable, recurrent, or metastatic tumors
Regulatory and Safety Profile:
- ATC Code: L01EA01
- Pharmacologic Class: BCR-ABL Tyrosine Kinase Inhibitor
- Pregnancy Category: D – Contraindicated unless essential
- Common Adverse Effects: Edema, nausea, rash, muscle cramps, fatigue
- Serious Adverse Effects: Hepatotoxicity, cytopenias, cardiotoxicity, severe fluid retention
- Contraindications: Hypersensitivity to imatinib or its excipients
- Monitoring Parameters: CBC, liver function, renal profile, serum electrolytes, and molecular response (BCR-ABL levels in CML)
For institutional pricing, pediatric oncology tender quotes, or regulatory documentation, please contact our commercial team at info@panamfarma.com.
You may also like…
Imatinib 400 mg 30 Tablet
Contact for QuoteImatinib
Marca de Patente: GLEEVEC
Clase: Es un inhibidor selectivo de tirosina quinasa (TKI)
Cómo Suministrado: -Pastillas orales de mesilato de imatinib: 400 mg 30 Tab
Clave: 010.000.4227.00
DESCRIPCIÓN:
Indicado para el tratamiento de diversas neoplasias hematológicas y tumores sólidos, incluyendo leucemia mieloide crónica (LMC) y tumores del estroma gastrointestinal (GIST).