Plasmanate – Plasma Protein Fraction 5% (Human)
Si Disponible: Dispensado al Día Siguiente
Envío Gratis
Dentro de México

Requiere Receta Médica
Este Medicamento requiere prescripción médica de su médico, clínica, hospital o terapeuta.
Plasmanate está indicado en el tratamiento del shock debido a quemaduras, lesiones por aplastamiento, emergencias abdominales y cualquier otra causa donde haya una pérdida predominante de líquidos plasmáticos y no de glóbulos rojos. También es eficaz en el tratamiento de emergencia del shock causado por hemorragias.
Description
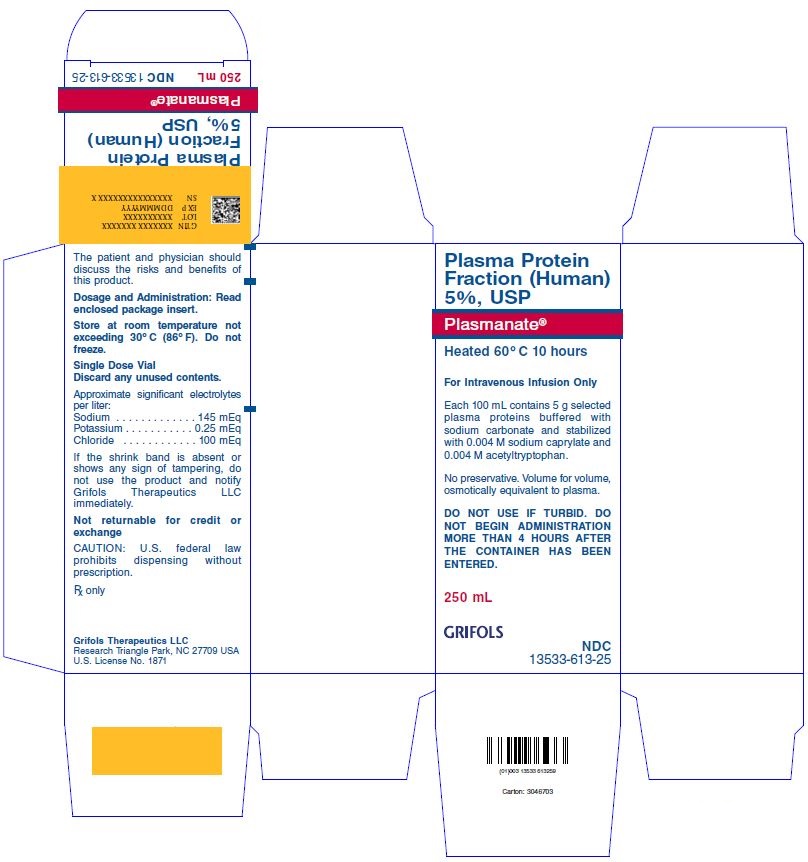
Plasmanate® (Plasma Protein Fraction [Human] 5%, USP)
Plasmanate® contains 5 g selected plasma proteins buffered with sodium carbonate and stabilized with 0.004 M sodium caprylate and 0.004 M acetyltryptophan.This product has been prepared from large pools of human plasma. Each 100 mL of Plasma Protein Fraction (Human) 5%, USP contains approximately 88% normal human albumin, 12% alpha and beta globulins, and not more than 1% gamma globulin as determined by electrophoresis. The concentration of these proteins renders this solution iso-oncotic with normal human plasma and isotonic. The approximate concentrations of the significant electrolytes in Plasmanate are:
- Sodium: 145 mEq/L
- Potassium: 0.25 mEq/L
- Chloride: 100 mEq/L
- Plasmanate is clear and amber-colored and must be administered intravenously.
Sterility and Viral Inactivation
This product is designed for use by the medical profession as a preparation derived from human blood, similar to human plasma. Each vial is sterile and heat-treated at 60°C for 10 hours to mitigate the possibility of transmitting hepatitis viruses.The blood group agglutinins and agglutinogens A and B are present at such low levels in Plasmanate that its use does not interfere with routine blood typing procedures. No chemical or microscopic alterations in urine have been observed with its administration.
The manufacturing process has been investigated for its ability to reduce infectivity of an experimental agent for transmissible spongiform encephalopathy (TSE), a model for variant Creutzfeldt-Jakob disease (vCJD) and classic CJD. Studies show that the steps from Pooled Plasma to Effluent IV-1 in Plasmanate production reduce TSE infectivity by ≥7.0 logs, providing reasonable assurance that if any vCJD/CJD agent were present, it would be effectively removed.
Indications and Usage
Treatment of Shock: Plasmanate is indicated in treating shock due to burns, crushing injuries, abdominal emergencies, and any cause of plasma fluid loss without significant red blood cell loss. It is also effective in emergency shock treatment due to hemorrhage. After the emergency phase, blood transfusion may be required based on the severity of blood loss. In infants and small children, Plasmanate is particularly effective for initial therapy of shock caused by dehydration and infection.Contraindications
- Contraindicated in patients on cardiopulmonary bypass due to risk of severe hypotension.
- Not to be used in patients with severe anemia, congestive heart failure, or increased blood volume.
How Supplied
Plasmanate is available in single-dose, rubber-stoppered vials in the following sizes:| NDC Number | Size | Grams Protein |
|---|---|---|
| 13533-613-20 | 50 mL | 2.5 |
| 13533-613-25 | 250 mL | 12.5 |
| 13533-613-27 | 500 mL | 25.0 |
Storage
Store at room temperature not exceeding 30°C (86°F).Package Label
Plasma Protein Fraction (Human) 5%, USP Plasmanate® Heated 60ºC for 10 hours For Intravenous Infusion Only Each 100 mL contains 5 g selected plasma proteins buffered with sodium carbonate and stabilized with 0.004 M sodium caprylate and 0.004 M acetyltryptophan. No preservative. Volume-for-volume, osmotically equivalent to plasma.*This is a Special Ordered Item and not always available in existing inventory, therefore plan ahead.
✕
Shopping cart0
There are no products in the cart!
Continue shopping
X
Products
Antibiotics
- Dactinomycin 500 µg - Cosmegen (Lyophilized powder for injection (IV) 500 µg/vial)
- Doxorubicin 50mg - Adriamycin, Rubex, Doxoruba (Injection solution (IV), lyophilized powder 50 mg)
- Doxorubicin 10mg - Adriamycin, Rubex, Doxoruba (Injection solution (IV), lyophilized powder 10 mg/mL)
- Doxorubicin, Liposomal 2mg - Doxil, Caelyx; (Liposomal IV dispersion 2 mg/mL in 10 mL vial)
Antibodies, monoclonal
- Trastuzumab 150mg - Herceptin (Vial, Intravenous infusion formulations—lyophilized pwd or sol. 150 mg)
- Trastuzumab 440mg - Herceptin (Intravenous infusion formulations—lyophilized powder or solution 440 mg)
- Bevacizumab 100mg - Avastin, Bevatas (IV Injection Vial 100 mg/4 mL)
- Bevacizumab 400mg - Avastin, Bevatas (IV Injection Vial 400 mg/16 mL)
- Brentuximab 50mg - Adcetris, Aark (IV lyophilized injection vials 50 mg/vial)
- Abciximab 10mg - ReoPro, Faximab (IV Injection Sol. 10 mg/5mL vial)
- Adalimumab 40mg/0.8mL - Humira, Amjevita, Imraldi, Cyltezo, Hyrimoz, Hulio, Hadlima, Abrilada, Cadalimab, Idacio, Simlandi (subcutaneous injection (solution) 40 mg/0.8 mL)
- Atezolizumab 840mg - Tecentriq (Intravenous injection (liquid, vial) 840 mg. /14mL per vial)
- Blinatumomab 35 µg - Blincyto (Intravenous lyophilized powder, single dose vial 35 µg)
- Cetuximab 100mg - Erbitux (originator), Cetuxa (biosimilar) (Intravenous solution (single-dose vial) 100 mg per vial (2 mg/mL))
- Adalimumab 80mg/0.8mL - Humira, Amjevita, Imraldi, Cyltezo, Hyrimoz, Hulio, Hadlima, Abrilada, Cadalimab, Idacio, Simlandi (PFS, Vial for subcutaneous injection (solution) 80 mg/0.8 mL)
- Denosumab 60mg pfs - Prolia®, Xgeva®; biosimilars: Jubbonti®, Wyost®, Ospomyv, Stoboclo (Subcutaneous injection solution (prefilled syringe or vial) 60 mg/mL)
- Denosumab 120mg - Prolia®, Xgeva®; biosimilars: Jubbonti®, Wyost®, (Subcutaneous injection solution (prefilled syringe or vial) 120 mg)
- Infliximab 100mg/mL (IV Injection Vial 100mg)
- Cetuximab 500mg - Erbitux (originator), Cetuxa (biosimilar) (Intravenous solution (single-dose vial) 500 mg per vial)
Antivirals - Antiretrovirals
- Abacavir/Lamivudine 600mg - Kivexa, Epzicom, Abec L, Abalam, ABEC‑L (30 Film-coated oral tablets 600 mg abacavir + 300 mg lamivudine per tablet)
- Abacavir 300mg - Ziagen®, Abamune, Trizivir (combination), Abec (Film-coated tablet 300mg)
- Darunavir + Cobicistat 800/150mg - Prezcobix® (US), Rezolsta® (EU) (Film-coated oral tablets 800 mg darunavir / 150 mg cobicistat per tablet)
- Dolutegravir 50 mg - Tivicay® (GSK), Instgra™ (Emcure) (Film‑coated oral tablets 50 mg per tablet)
- Dolutegravir + Abacavir + Lamivudine 600/50/300mg - Triumeq (30 Film-coated oral tablets 600 mg abacavir / 50 mg dolutegravir / 300 mg lamivudine per tablet)
- Doravirine 100 mg - Pifeltro® (30 Film‑coated oral tablets 100mg Tablets)
Asthma
- Benralizumab 30mg - Fasenra (PFS, Subcutaneous injection solutions in vials 30 mg 1 mL.)
- Iloprost 10 mcg/mL - Ventavis, Ilomedine, Aurlumyn (Inhalation solution (ampules) for nebulizer 10 µg/mL concentration in 1 mL ampules)
- Iloprost 20 mcg/mL - Ventavis, Ilomedine, Aurlumyn (Inhalation solution (ampules) for nebulizer 20 µg/mL concentration in 1 mL ampules)
Cardiovascular
- Enoxaparin sodium 20mg PFS - Lovenox, Clexane, Xaparin (PFS, Subcutaneous or Intravenous 20 mg/0.4mL)
- Enoxaparin sodium 40mg PFS - Lovenox, Clexane, Xaparin (PFS, Subcutaneous or Intravenous 40 mg/0.4mL)
- Enoxaparin sodium 60mg PFS - Lovenox, Clexane, Xaparin (PFS, Subcutaneous or Intravenous 60 mg/0.6 mL)
- Enoxaparin sodium 80mg PFS - Lovenox, Clexane, Xaparin (PFS, Subcutaneous or Intravenous 80 mg/0.8 mL)
Immuno Therapies
- Everolimus 2.5mg - Afinitor, Zortress, Certican, Votubia (Oral tablets 10 tab pack 2.5 mg)
- Peginterferon Alfa‑2a - Pegasys (Roche), Taspiance (Emcure), generics by Cipla, Emcure, etc (liquid solution for injection (vial) 180 µg per dose)
- Peginterferon Beta‑1a - Plegridy (Subcutaneous/intramuscular Injection; 125 µg per 0.5 mL dose)
- Etanercept 25mg Vial - Enbrel (originator); biosimilars: Benepali, Brenzys, Erelzi (Vial, Solution for subcutaneous injection 25 mg)
- Etanercept 50mg Vial - Enbrel (originator); biosimilars: Benepali, Brenzys, Erelzi (Vial, Solution for subcutaneous injection 50 mg)
Endocrine
- Letrozole 2.5 mg - Femara, Oncolet, Feofar, Zaronil, and others (Oral tablet 2.5 mg / 30 Tab)
- Anastrozole - Arimidex, Altraz, Anabrez (Oral Tablets (film-coated) 1 mg)
- Fulvestrant 250 mg/5 mL - Faslodex, Perjeta (Solution for intramuscular injection, vials containing 250 mg/5 mL 250 mg per 5 mL (50 mg/mL))
- Cabergoline 0.25 mg Tab - Dostinex, Cabgolin (Oral tablets 0.25 mg)
- Cabergoline 0.50 mg Tab - Dostinex, Cabgolin (Oral tablets 0.50 mg)
Vaccines
- CYD-TDV - Dengvaxia® (CYD‑TDV) (Live‑attenuated, injectable suspension Standard prefilled dose; one vial per dose)
- Haemophilus influenzae type B conjugate vaccine - BioHIB® (Lyophilized powder for reconstitution/injection (0.5 mL vial) 0.5 mL per vial;)
Insulin
- Insulin Glargine (Biosimilar) - Basalog, Basalog One, Basaglar, Lantus (Vial 100 IU\mL)
- Insulin Glargine (Lantus) - Lantus, Lantus SoloStar (10 mL vial 100 IU\/mL)
- Insulin Glargine (Glaritus) - Glaritus (Vial 10mL 100iu/mL standard long-acting)
- Insulin Lispro - Humalog (Vial 10mL 100iu/mL)
- Insulin Glargine - Basalog (Vial 5 mL 100 IU/mL standard concentration)
- PX-0915A, Insulin Glargine /recombinant human insulin analog - Lantus, Basaglar, Insolenta, Glargix-G (10 mL multi-dose 100 units/mL)
Antineoplastic / Cancer
- Dasatinib 70mg - Sprycel (Film-coated tablets 70 mg)
- Cabazitaxel 60mg - Jevtana (lyophilized IV powder 60 mg / 1.5 mL)
- Abiraterone Acetate 250 mg - Zytiga® (Oral Tablet, contain 120 tablets unless specified otherwise. 250 mg)
- Bendamustine 100mg/4mL - Treanda, Ribomustin, Cytostasan (Injectable, Lyophilized powder for intravenous injection (reconstitute) 100mg 4 mL)
- Carboplatin 450mg - Paraplatin, CARBOplatin, Novaplus (Intravenous injection – sterile aqueous solution, multi-dose vial 450 mg /45 mL)
- Bleomycin 15iu/15mg - Blenoxane (Lyophilized powder/injection, (IV, IM, SC, intrapleural) 15 IU. 15 mg)
- Cisplatin 50mg - Platinol-AQ, Platinol, Cytoplatin, Ciszest, Celplat‑50 (Intravenous injection (sterile aqueous solution, multi-dose vial) 50 mg/50 mL)
- Methotrexate 2.5mg Tab - Rheumatrex, Trexall (Tablets, Oral 100T 2.5 mg)
- Filgrastim 300 µg - Neupogen (Injection (IV/SC) Sol. 300 µg/mL)
- Imatinib 100 mg Tablet - Gleevec (Oral Tablets 100mg x 60 tab 100 mg)
- Imatinib 400 mg Tablet - Gleevec (Oral Tablets 400mg x 30 tab 400 mg)
- Methotrexate 500mg/20mL - Rheumatrex, Trexall (intravenous/intramuscular/subcutaneous injection 500 mg / 20 mL)
- Nilotinib 200mg Cap - Tasigna (100 Caps, Oral hard gelatin capsules 200 mg)
- Palonosetron 0.5mg - Aloxi (Oral Capsule 0.5 mg)
- Rituximab 100mg/10mL - Rituxan, Mabthera (Intravenous solution, concentrate for infusion 100 mg/10 mL)
- Rituximab 500mg/50mL - Rituxan, Mabthera (Intravenous solution, concentrate for infusion 500 mg/50 mL)
- Sorafenib 200mg Tab - Nexavar (Oral film-coated tablets 200 mg)
- Sunitinib 12.5mg Cap - Sutent (Oral hard gelatin capsules 12.5 mg)
- Mercaptopurine (6‑MP) - Purinethol, Purixan, Xaluprine (Oral tablets 50 mg. 100 Tab)
- Vincristine 1mg/10mL - Oncovin, Vincasar (Intravenous injection 1 mg / 1 mL)
- Fluorouracil 500 mg/10 mL - Adrucil (Injectable, single-dose vial (SDV) 500 mg/ 10 mL)
- Fluorouracil 1 gm/20 mL - Adrucil (Injectable, single-dose vial (SDV) 1 gm/20 mL)
- Fluorouracil 50 mg/mL - Adrucil (Injectable, multiple-dose vial (MDV) 50 mg/mL)
- Carboplatin 50mg/5 mL - Carbokem (Alkem) (Injectable, multiple-dose vial 50 mg in 5 mL (10 mg/mL))
- Carboplatin 600 mg/60 mL - Carbokem (Alkem) (Injectable, single- or multiple-dose vial 600 mg in 60 mL (10 mg/mL))
- Cisplatin 10 mg/10 mL - Cislieva, Kemoplat, Platinex (Aqueous IV injection, vial 10 mg in 10 mL (1 mg/mL))
- Paclitaxel 30 mg/5 mL - Pacliltax, Nanoxel, Z-Taxel, Altaxel, Plaxel (Injectable, multidose vial 30 mg in 5 mL (6 mg/mL))
- Paclitaxel 100 mg/16.7 mL - Pacliltax, Nanoxel, Z-Taxel, Altaxel, Plaxel (Intravenous Multidose vial, Includes solution/albumin‑bound pwdr for reconstitution 100 mg in 16.7 mL (6 mg/mL))
- Paclitaxel 260 mg/43.4 mL - Pacliltax, Nanoxel, Z-Taxel, Altaxel, Plaxel (Injectable, multidose vial 260 mg in 43.4 mL (6 mg/mL) Vial)
- Methotrexate 1gm/10mL - Rheumatrex, Trexall (Injectable, multidose vial 1 gm/10 mL 25mg/mL)
- Carfilzomib 60mg - Kyprolis (Lyophilized IV powder for solution 60 mg)
- Hydroxyurea 500 mg (Hydroxycarbamide) - Hydrea, Droxia, Siklos, Xromi, Tharolax, Hondrea, Oxyrea, Cytodrox, Myelostat, (Oral capsule/tablet 500 mg capsules/tablets)
- Aflibercept 100mg - EYLEA®, Zaltrap (Single-dose vials for intravenous or intravitreal use 100 mg/4 mL (25 mg/mL) S)
- Everolimus 5mg - Afinitor, Zortress, Certican, Votubia (Oral tablets 10 tab pack 5 mg.)
- Everolimus 10mg - Afinitor, Zortress, Certican, Votubia (Oral tablets 10 tab pack 10 mg.)
- Mitoxantrone 2mg/mL - Novantrone (original), Sun’s Oncotron, VHB’s Mitozan (India) (Intravenous solution; lyophilized powder for injection (vial) 2mg/mL)
- Lomustine 100mg - Gleostine, CCNU, CeeNu, Ceenu, Lomuwin, Lomoother, Belustine (Oral hard gelatin capsules /100 Cap Package 100 mg)
- Lomustine 40mg - Gleostine, CCNU, CeeNu, Ceenu, Lomuwin, Lomoother, Belustine (Oral hard gelatin capsules /100 Cap Package 40 mg)
- Dasatinib 100mg - Sprycel (Film-coated tablets 100 mg)
- Dasatinib 140mg - Sprycel (Film-coated tablets 140 mg)
- Dasatinib 20mg - Sprycel (Film-coated tablets 20 mg)
- Pemetrexed disodium 500mg - Alimta, Pemrydi RTU, Pemnat, Pemgem, Pexotra (Intravenous injection, sterile solution in single-dose vial 500 mg/20 mL (25 mg/mL))
- Amifostine - Ethyol (Clinigen) (Powder for intravenous injection (tri-hydrate/API) 500 mg)
- Oxaliplatin 50mg - Eloxatin, Oxitan (Inj Vial, powder for reconstitution 50mg / 50 mL)
- Irinotecan 100mg - Campto® (Pfizer), Irinotel® (Inj Vial 100 mg)
- Etoposide 50mg Cap - Vepesid®, Floposid®, Etopophos® (Oral Capsule 50 mg)
- Etoposide 20mg - Vepesid®, Floposid®, Etopophos® (Intravenous injection 20 mg)
- Oxaliplatin 100mg - Eloxatin, Oxitan (Inj Vial, powder for reconstitution 100mg / 20 mL)
- Irinotecan 40mg - Campto® (Pfizer), Irinotel® (Inj Vial 40 mg)
- Etoposide 1g. - Vepesid®, Floposid®, Etopophos® (Intravenous injection 1gm/50 mL)
- Etoposide 500mg - Vepesid®, Floposid®, Etopophos® (Intravenous injection 500 mg/25 mL)
- Etoposide 100mg - Vepesid®, Floposid®, Etopophos® (Intravenous injection 100 mg/5 mL)
- Etoposide 100mg Tab - Vepesid®, Floposid®, Etopophos® (Oral Tablet 100 mg)
- Daunorubicin 20mg - Daunohal; Cerubidine; Daunomycin; Daunotec; Dauneon; Daunomac; Vyxeos (liposomal form); DaunoXome (Lyophilized powder for IV injection; vial form 20 mg per vial (≈21.4 mg hydrochloride salt equivalent))
- Dacarbazine 100mg/20mL - DTIC‑Dome; Bazipar; Cedcozine; Dacarba; Dacarex; Dacin; Dacmed; Darbazine; Dazine; (Powder for injection (IV) 100 mg)
- Trastuzumab emtansine 100mg (ado‑trastuzumab emtansine; T‑DM1) - Kadcyla® (Sterile lyophilized powder for concentrate for infusion (single‑dose vial) 100mg)
- Trastuzumab emtansine 160mg (ado‑trastuzumab emtansine; T‑DM1) - Kadcyla® (Sterile lyophilized powder for concentrate for infusion (single‑dose vial) 160 mg)
- Imatinib 80 mg/mL Oral Sol. - Gleevec (Oral Sol 80 mg/mL of 240 mL 80 mg/mL)
- Rituximab Hycela 1,400mg/23,400U SC - Rituxan, Mabthera (Intravenous solution, concentrate for infusion 1,400 mg rituximab / 23,400 units hyaluronidase per 11.7 mL, Sub-C)
- Rituximab Hycela 1,600mg/26,800U 13.4mL - Rituxan, Mabthera (Sub-C 1,600 mg rituximab / 26,800 units hyaluronidase per 13.4 mL, Sub-C)
- Dasatinib 50mg - Sprycel (Film-coated tablets 50 mg)
- Dasatinib 10mg/mL Oral Suspension - Sprycel (10mg/mL Powder for Oral Suspension (pediatric) 10 mg /mL.)
- Nilotinib 50mg Cap - Tasigna (Hard gelatin capsules 50 mg)
- Nilotinib 71mg Tabs - Tasigna (Tablets 71 mg)
- Nilotinib 95mg Tabs - Tasigna (Tablets, Oral 71 mg)
- Methotrexate 5mg Tab - Rheumatrex, Trexall (Tablets, Oral 5 mg)
- Methotrexate 10mg Tab - Rheumatrex, Trexall (Tablets, Oral 10 mg)
- Methotrexate 10mg/0.4mL - Rheumatrex, Trexall (10 mg/0.4 mL Sub-C 10 mg/0.4 mL)
- Methotrexate 25 mg/1 mL Sub-C - Rheumatrex, Trexall (Inj Vial, Sub-C 25 mg/1 mL)
- Methotrexate 15mg Tab - Rheumatrex, Trexall (Tablets, Oral 15 mg)
- Vincristine 2mg/2mL - Oncovin, Vincasar (Single-dose vial, Intravenous use only 2mg/ 2mL)
- Pertuzumab 420mg/14mL - Perjeta (Vial, Concentrate for intravenous infusion 420 mg/14 mL vial (30 mg/mL))
- Nivolumab 40mg/4mL - Opdivo (Intravenous solution vial; 10 mg/mL concentration 40 mg/4 mL)
- Nivolumab 100mg - Opdivo (Intravenous solution vial; 100 mg/mL)
- Paclitaxel 300mg/50mL - Taxol, Pacliltax, Nanoxel, Z-Taxel, Altaxel, Plaxel (Concentrate for infusion (vial) 300 mg/50 mL (6 mg/mL)
- Pembrolizumab 100mg/4mL - Keytruda (Intravenous solution, vial (concentrate for infusion) 100 mg/4 mL (25 mg/mL))
- Mitomycin 10mg (Mitomycin C) - Mutamycin, Jelmyto, Zusduri, Mitosol (Intravenous injection (lyophilized powder vial) 10 mg vial)
- Leucovorin, Calcium - Biovorin (Parenteral injection (liquid solution/ampoules/vials) 50 mg/5 mL)
Biologics / Biopharmaceuticals
- Peginterferon Alfa‑2b - ViraferonPeg, PegIntron (Injectable solution – vials 150 μg vial / 1.5 μg/kg)
- Erythropoietin alpha 4000iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 4,000 IU/mL)
- Erythropoietin alpha 6000iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 6,000 IU/mL)
- Erythropoietin alpha 2000iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 2,000 IU/mL)
- Erythropoietin alpha 10,000 iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 10,000 IU/mL)
- Erythropoietin alpha 20,000 iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 20,000 IU/mL)
- Erythropoietin alpha 40,000 iu - EpoGen, Procrit, Retacrit (biosimilar), Epoetin Alfa Hexal (Solution for inj (vial, pre‑filled syringes) 40,000 IU/mL)
Antidiabetic Agents
Blood Products / Hemoderivatives
- Albucel 5% - Human Albumin Solution IP/EP (Solution for IV infusion 5% w/v human albumin)
- Albucel 20% - Human Albumin Sol IP/EP (Solution for IV infusion 20% w/v albumin)
- Albucel 20% - Human Albumin Sol IP/EP (Solution for IV infusion 20% w/v albumin)
- Albucel LS - Human Albumin Sol. (Low Salt) (Solution (likely IV, “LS” = low-sodium) Low-sodium variant; strength same as 5% or 20%)
- Celesterase 1,500iu - Human C1-Esterase Inhibitor EP 500 IU (Injection (vial) 500 IU C1-esterase inhibitor)
- Factocel IX 250iu - Freeze Dried Human Coagulation Factor IX IP/EP (Injection (vial) 250 IU / 600 IU Factor IX)
- Factocel VIII 500iu - Dried Human Antiheamophillic Factor VIII IP/EP (Injection (vial) 250 IU & 500 IU Factor VIII)
- Factocel VIII 500iu - Dried Human Antiheamophillic Factor VIII IP/EP (Injection (vial) 500 IU)
- Fibrogen-I 0.5g - Freeze Dried Powder Human Fibrinogen EP (Injection (vial) 0.5 g human fibrinogen)
- Fibrogen-I 1g - Freeze Dried Powder Human Fibrinogen EP (Injection (vial) 1 g human fibrinogen)
- Globucel 5% 5g - Human Normal Immunoglobulin for Intravenous Use Sol IP/EP (100 mL vial for IV infusion 5 g/100 mL human immunoglobulin (5%))
- Globucel 10% 10mL - Human Normal Immunoglobulin for Intravenuose Use Solution IP/EP (Solution for infusion (vial) 10 mL)
- Globucel 10% 50mL - Human Normal Immunoglobulin for Intravenuose Use Solution IP/EP (Solution for infusion (vial) 50 mL)
- Globucel 10% 100mL - Human Normal Immunoglobulin for Intravenuose Use Solution IP/EP (Solution for infusion (vial) 100 mL)
- Globucel-SC 16.5 - Human Normal Immunoglobulin for Subcutaneous Use Solution IP/EP (Vial 16.5% IgG (10 mL vial))
- Globucel-VF 5g - Human Normal Immunoglobulin for Intravenous Use Solution IP/EP (Vial 5 g/100 mL (5%))
- Globucel-VF 10g - Human Normal Immunoglobulin for Intravenous Use Solution IP/EP (Vial 10 g/100 mL (10%))
- Globucel 5% - Human Normal Immunoglobulin for Intravenous Use Solution IP/EP (Vial 5 g/100 mL (5%))
- Intaglob 2mL - Human Normal Immunoglobulin for Intramuscular Use Solution IP/EP (Injection (vial) – Human immunoglobulin 2 mL)
- Pro-Throm - Freeze Dried Powder Human Prothrombin Complex IP (Injection (vial) – Prothrombin complex concentrate 250 IU)
- Darbepoetin alfa 25 µg - Aranesp (Injection (solution) 25 µg)
- Darbepoetin alfa 40 µg - Aranesp (Injection (solution) 40 µg)
- Darbepoetin alfa 60 µg - Aranesp (Injection (solution) 60 µg)
- Darbepoetin alfa 200 µg - Aranesp (Injection (solution) 200 µg)
- Darbepoetin alfa 100 µg - Aranesp (Injection (solution) 100 µg)
- Pegfilgrastim 6 mg - Neulasta, Fulphila, Pelgraz (Solution for subcutaneous injection, Vial 6 mg per 0.6 mL)
- Romiplostim 125 µg - Nplate, Romy (Powder for reconstitution; solution for subcutaneous injection, Vial 125 µg per vial)
- Romiplostim 250 µg - Nplate, Romy (Powder for reconstitution; solution for subcutaneous injection, Vial 250 µg per vial)
- Romiplostim 500 µg - Nplate, Romy (Powder for reconstitution; solution for subcutaneous injection, Vial 500 µg per vial)
Osteo
- Teriparatide 600 mcg - Forteo, Forsteo, Biosimilars... (PFS & Vial, solution for subcutaneous injection 600 mcg per 2.4 mL pen (250 mcg/mL; delivers 28 daily doses of 20 mcg))
- Teriparatide 750 mcg - Forteo, Forsteo, Biosimilars... (PFS & Vial, solution for subcutaneous injection 750 mcg)
Other
- Docetaxel 20mg - Taxotere (IV concentrate/injection vials 20 mg)
- Cytarabine 100mg - Cytosar-U (Injectable solution for IV 100 mg/5 mL)
- Cytarabine 500mg - Cytosar-U (Injectable solution for IV 500 mg/25 mL)
- Cytarabine 2gm - Cytosar-U (Injectable solution for IV 2 g./ 20mL)
- Docetaxel 80mg - Taxotere (IV concentrate/injection vials 80 mg)
- Carboplatin 150mg - Paraplatin (IV powder for infusion 150 mg)
- Carfilzomib 10mg - Kyprolis (Lyophilized IV powder for solution 10 mg)
- Carfilzomib 30mg - Kyprolis (Lyophilized IV powder for solution 30 mg)
- Carmustine 100mg - BiCNU (IV powder for infusion 100 mg)
- Bortezomib 2 mg - Velcade (Lyophilized powder/injection (IV or SC). 2 mg)
- Bortezomib 3.5 mg - Velcade (Lyophilized powder/injection (IV or SC). 3.5 mg)
- Bortezomib 1 mg - Velcade (Lyophilized powder/injection (IV or SC). 1 mg)
- Cisplatin 100mg - Platinol-AQ (Intravenous solution 100 mg/100 mL)
- Cisplatin 200mg - Platinol-AQ (Intravenous solution 200 mg/200 mL)
- Cyclophosphamide 500mg/2.5mL - Cytoxan (IV solution 500 mg/2.5 mL)
- Cyclophosphamide 1g/5mL - Cytoxan (IV solution 1 g/5 mL)
- Cyclophosphamide 25mg - Cytoxan (Oral Capsules 25 mg)
- Cyclophosphamide 50mg - Cytoxan (Oral Capsules 50 mg)
- Docetaxel 120mg - Taxotere (IV concentrate/injection vials 120 mg)
- Dutasteride 0.5mg - Avodart (Oral Soft Gelatin Capsules 0.5 g capsules)
- Filgrastim 480 µg - Neupogen (Injection (IV/SC) Sol. 480 µg/1.6 mL)
- Gemcitabine 200mg - Gemzar (IV Lyophilized powder for infusion 200 mg)
- Gemcitabine 1gm - Gemzar (IV Lyophilized powder for infusion 1 gm)
- Mesna 200mg - Mesnex, Uromitexan (Intravenous Sol. 200 mg/2 mL)
- Mesna 400mg - Mesnex, Uromitexan (Intravenous Sol. 400 mg/4 mL)
- Mesna 1gm - Mesnex, Uromitexan (Intravenous Sol. 1 g/10 mL)
- Mesna 400mg Tab - Mesnex, Uromitexan (Oral Tablets 400 mg)
- Methotrexate 25mg/mL - Rheumatrex, Trexall (intravenous/intramuscular/subcutaneous injection 25 mg/mL)
- Methotrexate 50mg/2mL - Rheumatrex, Trexall (intravenous/intramuscular/subcutaneous injection 50 mg/2 mL)
- Methotrexate 40mL - Rheumatrex, Trexall (intravenous/intramuscular/subcutaneous injection 40 mg/mL)
- Nilotinib 150mg - Tasigna (Oral hard gelatin capsules 150 mg)
- Ondansetron 8mg/4mL - Zofran (Injection (IV/IM) 2 mg/mL)
- Ondansetron 4mg Tab - Zofran (Oral Tablets 4 mg)
- Ondansetron 8mg - Zofran (Oral tablets 8 mg)
- Ondansetron 24mg - Zofran (Oral tablets 24 mg)
- Palonosetron 0.25mg/2mL - Aloxi (IV injection 0.25 mg/2mL)
- Palonosetron 0.25mg/5mL - Aloxi (IV injection 0.25 mg/5mL)
- Pegaspargase 3.75iu - Oncaspar (Intravenous or intramuscular injection: liquid solution & lyophilized powder for injection 3,750 IU /5 mL)
- Sunitinib 25mg - Sutent (Oral hard gelatin capsules 25 mg)
- Sunitinib 37.5mg - Sutent (Oral hard gelatin capsules 37.5 mg)
- Sunitinib 50mg - Sutent (Oral hard gelatin capsules 50 mg)
- Temozolamide 100mg/mL - Temodar (Injection (IV/IM) 100 mg)
- Temozolamide 5mg - Temodar (Oral capsules 20 mg)
- Temozolamide 20mg - Temodar (Oral capsules 20 mg)
- Temozolamide 100mg - Temodar (Oral capsules 100 mg)
- Temozolamide 140mg - Temodar (Oral capsules 140 mg)
- Temozolamide 180mg - Temodar (Oral capsules 180 mg)
- Temozolamide 250mg - Temodar (Oral capsules 250 mg)
- Trastuzumab 600mg - Herceptin (Intravenous infusion formulations—lyophilized powder or solution 600 mg)
- Vinblastine 10mg - Velban (Intravenous injection solution 10 mg/10mL)
×
Detalles de producto
Artículo
Cantidad

Loading...
Catálogo de medicamentos